Opportunities
The pharmaceutical and life science industry historically represents one of the most successful economic industries. Approximately 2 billion prescriptions are filled each year in the US alone. However, drug discovery and development is a long and complex process, averaging 10-15 years and $1.8 billion per drug from discovery to market. Small molecule drugs (NCEs) and biologics (NBEs) are two major categories of drugs in the current market. Small molecule drugs bind well to the protein target but are notoriously expensive to discover. It takes years and the synthesis of tens to millions of research compounds before a lead can be discovered. Once a lead is found, there is no guarantee that the drug will get to market. Most small molecule drugs fail clinical trials due to toxicity caused by non-specific (off target) interactions with other proteins. Biologic therapeutics, such as vaccines, blood, gene, antibodies, or protein therapeutices tend to have a shorter discovery phase, but longer overall development time. Once a protein target is identified, an antibody that targets this protein can be identified and developed as a drug. Biologics, such as proteins, antibodies, and gene carriers, suffer poor bioavailability due to stability issues and tend to not be very effective for treatment.
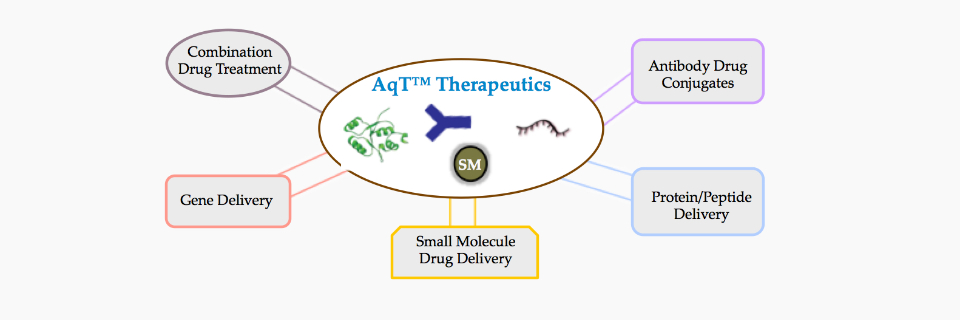
Our game-changing plan for developing a safe and effective treatment for patients is to develop a hybrid drug from the right combination of small molecule drugs, biologics, and our specifically designed AqT™ molecules. Because the individual drug does not need to meet all of the criteria for a safe and effective treatment if other conjugated components have such properties, this approach can greatly shorten the drug development process. However, for this hybrid approach to be successful, the conjugation process for preparing such a hybrid drug and the linker/carrier used should not affect the properties of the individual drug components after conjugation or the individual drugs being released fully in an active form. We have already built and optimized such advanced conjugation processes. In addition, if the linker/carrier can introduce new properties and add beneficial effects to the drugs that are conjugated, as is the case with AqT™ molecules, then it will greatly improve the therapeutic profile of a hybrid drug.
AqT™ drug classes